- Remdesivir and FIP: Treatment Results in Feline Infectious Peritonitis (FIP)
- Introduction - Remdesivir and FIP
- Overview of FIP and its treatment - Remdesivir and FIP
- Study results on the effectiveness of treatment - Remdesivir and FIP
- Practical use and dosage - Remdesivir and FIP
- Important findings for practice - Remdesivir and FIP
- Explanation of GC-376 and GS-441524 with brand names
- GC-376
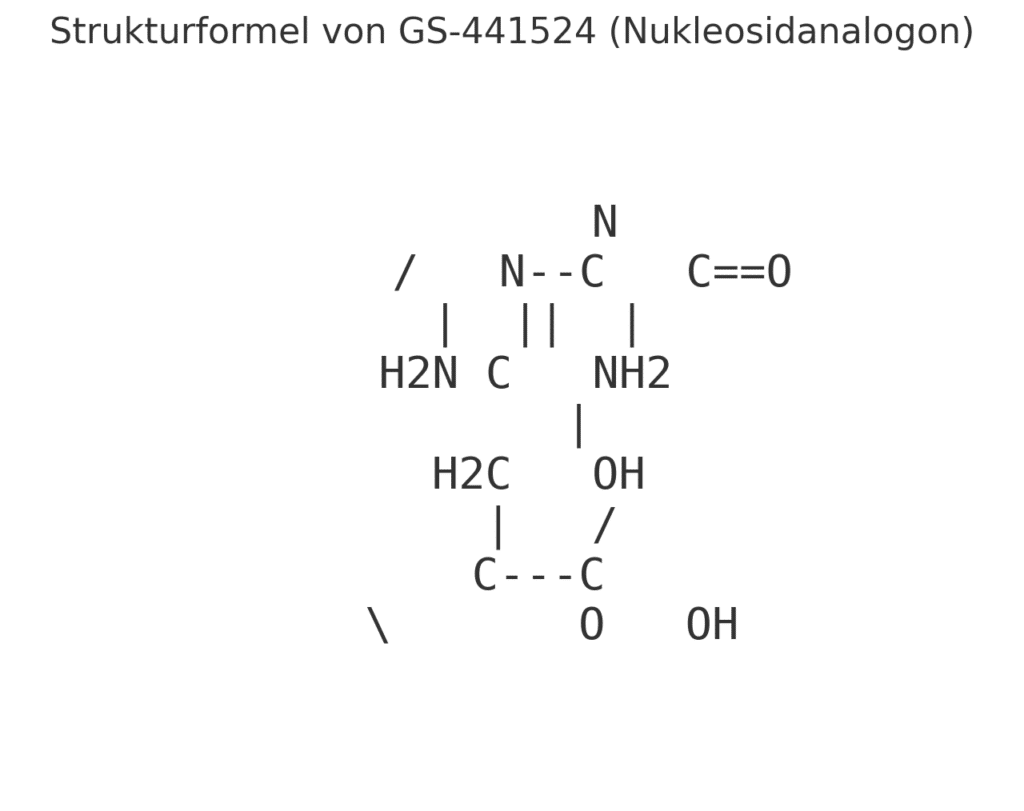
- GS-441524
- Association between GS-441524 and remdesivir and FIP
- GS-441524: The Foundation
- Remdesivir: The big brother
- Transition from remdesivir to GS-441524
- Frequently asked questions (FAQ) about treating FIP with GS-441524 and remdesivir
- Are GS-441524 and Remdesivir approved in Germany for the treatment of FIP in cats?
- Introduction - Remdesivir and FIP
- Remdesivir and FIP: How they work
- Studies and clinical experiences - Remdesivir and FIP
- Treatment protocols - Remdesivir and FIP
- Challenges and legal aspects - Remdesivir and FIP
- Side effects and risks - Remdesivir and FIP
- Success Stories - Remdesivir and FIP
- Future prospects - Remdesivir and FIP
- Conclusion - Remdesivir and FIP
Remdesivir and FIP: Treatment Results in Feline Infectious Peritonitis (FIP)
Introduction - Remdesivir and FIP
Feline Infectious Peritonitis (FIP) has long been considered a fatal disease in cats. However, the discovery of the drugs GC-376 and GS-441524 has significantly improved the prognosis for affected cats. This article presents the latest findings on the effectiveness of these treatment options and their practical application.
Overview of FIP and its treatment - Remdesivir and FIP
FIP is a viral disease caused by the feline coronavirus. The disease occurs in two main forms: the wet form, characterized by abdominal or thoracic effusions, and the dry form, in which granulomatous changes occur in various organs. Until recently, FIP was fatal in almost all cases because there was no effective treatment.
Discovery of GC-376 and GS-441524
GC-376 and GS-441524 are antiviral drugs that have been shown to be effective against FIP. GC-376 inhibits the viral protease and thus prevents the virus from multiplying. GS-441524, a nucleoside analogue, works by incorporating itself into viral RNA and stopping it from replicating.
Study results on the effectiveness of treatment - Remdesivir and FIP
A recent study examined the treatment of 28 cats with FIP using remdesivir (a prodrug of GS-441524) and its oral form. Treatment lasted at least 84 days and included intravenous remdesivir (10 mg/kg daily for 4 days, followed by 6 mg/kg subcutaneously daily). Cats that had difficulty with the injections were later given oral GS-441524 tablets.
Treatment outcomes and survival rate - Remdesivir and FIP
The study found that 86% of cats survived after six months. The survival rate increased to 96% when cats that died within the first 48 hours were excluded. The relapse rate within six months was 30% in the low-dose remdesivir group, while there were no relapses in the high-dose group and the group that switched to oral GS-441524.
Practical use and dosage - Remdesivir and FIP
The optimal dosage and duration of treatment have not yet been definitively established, but may be 10 to 15 mg/kg daily for 84 days or longer. Both oral and injectable forms of the medication have been shown to be effective.
Important findings for practice - Remdesivir and FIP
- High efficacy : GC-376 and GS-441524 can achieve a cure rate of over 85% in cats with FIP.
- Rapid clinical response : Clinical and laboratory response is often rapid and a good predictor of long-term success.
- Different dosage forms : Both the oral and injectable forms of the medication are effective.
- Ongoing Research : The ideal dosage, route of administration, combination or adjunctive therapies, and transition schedules are not yet fully determined and remain the subject of active research.
- Legal and ethical considerations : In many countries, these medications are difficult to obtain due to legal ambiguities. The legal and ethical implications should be discussed with animal owners.
The treatment of FIP with GC-376 and GS-441524 represents a significant advance in veterinary medicine. These medications offer affected cats a real chance of recovery and significantly improve their quality of life. Continued research will help further optimize treatment protocols, further increasing the chances of survival for cats with FIP.
Explanation of GC-376 and GS-441524 with brand names
GC-376
Description and mode of action
GC-376 is a protease inhibitor specifically designed to inhibit the proliferation of Feline Coronavirus (FCoV), which is the cause of FIP. By inhibiting the main viral protease, GC-376 prevents the virus from replicating, thereby reducing the viral load in the cat's body.
Brand name
GC-376 is known under the brand name Xraphconn Xraphconn has made a name for itself in the veterinary community due to its effectiveness and good tolerability.
GS-441524
Description and mode of action
GS-441524 is a nucleoside analogue and works by incorporating into the viral RNA and stopping the virus from replicating. This drug is a prodrug of remdesivir, which was originally developed to treat Ebola and later COVID-19 in humans.
Brand name
GS-441524 is known by the brand name Mutian . Mutian has been established as an effective and well-tolerated treatment option for cats with FIP. It offers a flexible treatment option in both injectable and oral forms.
Association between GS-441524 and remdesivir and FIP
The antiviral drugs GS-441524 and remdesivir have proven to be particularly effective in the treatment of feline infectious peritonitis (FIP). Both drugs are closely related and come from the same pharmacological family. This article discusses the connection between GS-441524 and remdesivir and their importance in the treatment of FIP in cats.
GS-441524: The Foundation
Mode of action
GS-441524 is a nucleoside analogue that is incorporated into the viral RNA and inhibits the replication of the virus. It is the active metabolite of remdesivir and acts directly against the feline coronavirus (FCoV), which causes FIP. In clinical trials, GS-441524 has demonstrated high efficacy and good tolerability in the treatment of FIP.
Availability and Usage
GS-441524 is known by the brand name Mutian and is commonly used in veterinary medicine. It is available in injectable and oral forms, allowing flexibility in treatment.
Remdesivir: The big brother
Mode of action
Remdesivir is a prodrug that is converted into GS-441524 in the body. It was originally developed to treat Ebola and was later used to treat COVID-19 in humans. Remdesivir inhibits the virus's RNA polymerase, effectively stopping the virus from reproducing.
Use in cats
In the treatment of FIP in cats, remdesivir is administered intravenously and shows similar efficacy to GS-441524. Intravenous administration of remdesivir may be particularly useful when rapid and intensive treatment is required.
Transition from remdesivir to GS-441524
Treatment protocol
In many cases, treatment of FIP is started with an initial dose of remdesivir. Treatment often starts with intravenous administration of remdesivir to achieve a rapid reduction in viral load. After this initial phase, treatment is often switched to oral administration of GS-441524 to continue therapy and prevent viral replication in the long term.
Frequently asked questions (FAQ) about treating FIP with GS-441524 and remdesivir
What is Feline Infectious Peritonitis (FIP) and how is it diagnosed?
Feline Infectious Peritonitis (FIP) is a serious, often fatal disease in cats caused by the feline coronavirus (FCoV).
There are two main forms of FIP: the wet form, which is characterized by fluid accumulation in the abdominal or chest area, and the dry form, which involves granulomatous inflammation in various organs. Both forms cause serious health problems and are usually fatal without treatment. Diagnosis of FIP is complex and requires a combination of clinical signs, laboratory findings, and specialized tests.
Diagnostic methods include: Clinical signs : These include fever, weight loss, loss of appetite, fatigue, jaundice and eye changes.
Laboratory findings : Blood tests may show increased globulin levels, decreased albumin-globulin ratios, and inflammatory signs.
Imaging procedures : Ultrasound examinations can reveal fluid accumulations or organ changes.
Specialized tests : PCR (polymerase chain reaction) and immunocytochemistry are important for directly detecting the virus and confirming the diagnosis.
A definitive diagnosis is often made by a combination of these methods, as no single method is completely certain.
How does GS-441524 and Remdesivir work in treating FIP?
GS-441524 and remdesivir are antiviral medications that have been shown to be effective in treating FIP.
Both drugs work by inhibiting viral replication, but they have different mechanisms and modes of use. GS-441524 : This drug is a nucleoside analog and the active metabolite of remdesivir. It is incorporated directly into the viral RNA, stopping the virus from replicating. GS-441524 is known under the brand name Mutian and can be administered both orally and by injection.
Treatment usually takes place over a period of at least 84 days. Remdesivir : Remdesivir is a prodrug that is metabolized in the body to GS-441524.
It was originally developed to treat Ebola and later to treat COVID-19 in humans. In veterinary medicine, remdesivir is administered intravenously and may be particularly useful in acute cases of FIP. The initial treatment with remdesivir can then be supplemented with oral administration of GS-441524. Both drugs have been shown to significantly improve the chances of survival for cats with FIP and are often used in combination to achieve the best therapeutic effect.
What side effects can occur when treated with GS-441524 and Remdesivir and FIP?
As with all medications, side effects can occur when treated with GS-441524 and remdesivir.
However, these are usually rare and usually mild. Known side effects include: Local injection site reactions : These include swelling, redness and pain that may occur with subcutaneous administration.
These symptoms are usually temporary and go away after a few days. Digestive problems : Some cats may develop diarrhea or vomiting during oral treatment with GS-441524.
These symptoms are usually mild and temporary. Liver and kidney values : In some cats, liver and kidney values may change during treatment.
Regular blood tests are important to monitor these parameters and adjust dosage if necessary. Loss of appetite and tiredness : Some cats may temporarily eat less and feel more tired than usual during treatment.
These symptoms should be monitored closely to make sure they do not indicate a more serious side effect. It is important that pet owners work closely with their veterinarian to monitor their cat's health during treatment and take immediate action if side effects occur.
How long does it take to treat FIP with these medications and when do you first see improvements?
Treatment of FIP with GS-441524 and remdesivir is usually lengthy and can last at least 84 days.
The exact duration of treatment depends on the severity of the disease and the cat's response to therapy. Initial phase of treatment : Intravenous injections of remdesivir are often given in the first few days to weeks of treatment, especially in severe cases or cats that need to respond quickly.
Transition phase : After initial stabilization, oral administration of GS-441524 is often switched to.
This phase usually lasts several weeks. Long-term treatment : To ensure the virus is completely controlled, treatment is often continued for several months.
Regular veterinary checks and blood tests are necessary to adjust therapy and monitor the cat's health. The first improvements can be visible in the first few days of treatment. These include a reduction in fever, improved appetite and increased activity. In many cases, the cat gains weight again and shows a significant improvement in its general condition within the first two weeks.
Where can I get GS-441524 and Remdesivir to treat my cat and how much does it cost?
GS-441524 and remdesivir are specialized medications and are not readily available in all countries.
Here is some important information about procurement and costs: Procurement : The availability of these medications may vary from country to country.
In some countries they are only available through special veterinary prescriptions, while in other countries they may not be legally available. It is important to consult with an experienced veterinarian who is familiar with treating FIP and has access to these medications. Cost : The cost of treating FIP with GS-441524 and remdesivir can be significant.
A full treatment can cost several thousand euros, depending on the dosage and duration of treatment. The costs consist of the medication itself, the veterinary services and the regular blood tests to monitor the treatment. Experienced Veterinarian : It is advisable to seek out a veterinarian who has experience treating FIP.
Such vets can often have better sources for obtaining medication and can provide sound advice. In summary, treating FIP with GS-441524 and remdesivir is a significant investment, but one that can significantly improve the survival and quality of life of affected cats. Close collaboration with a specialized veterinarian is the key to the success of this therapy.
Are GS-441524 and Remdesivir approved in Germany for the treatment of FIP in cats?
GS-441524
GS-441524 is currently not officially approved in Germany for the treatment of FIP in cats. However, it is often prescribed by veterinarians through what is known as “off-label use.” This means that veterinarians can use the drug to save cats' lives despite the lack of official approval. In some cases, a special import permit may be requested to import GS-441524 from abroad ( American Animal Hospital Association ) ( FIP Treatment Guide ).
Remdesivir
Remdesivir is approved in Germany as a drug to treat COVID-19 in humans. It is also used off-label for the treatment of FIP in cats. Remdesivir can be administered intravenously, especially in acute cases or if the cat cannot tolerate oral medications. However, there is no specific veterinary approval for remdesivir to treat FIP, which is why veterinarians require informed consent from pet owners before prescribing it ( Epicur Pharma® ) ( FIP Treatment Guide ).
Practical steps for cat owners - Remdesivir and FIP
- Advice from a specialized veterinarian : Seek out a veterinarian who has experience treating FIP. These professionals can provide better information about the latest developments and alternative procurement options.
- Import Authorization : Consider requesting an import authorization for GS-441524 with your veterinarian to legally obtain the drug from abroad.
- Off-label use : Find out about the possibilities and risks of “off-label” use of remdesivir and GS-441524 by your veterinarian.
While GS-441524 and remdesivir are not officially approved in Germany to treat FIP in cats, there are still ways affected cats can gain access to these life-saving medications. Close collaboration with experienced veterinarians and careful consideration of legal and health aspects are crucial. Through well-founded information and targeted measures, cat owners can do the best for the health of their beloved animals ( BOVA GLOBAL ) ( American Animal Hospital Association ) ( FIP Treatment Guide ).
“Off-label use” means that a drug is used outside of its officially approved indications. This means that the drug is used for a different disease, in a different dosage or for a different patient group than that specified in the approval by the relevant health authority (such as the EMA in Europe or the FDA in the USA).
Examples of off-label use - Remdesivir and FIP
- Other Disease : A drug originally developed to treat one disease is used to treat another disease. For example, remdesivir, originally approved for Ebola and later for COVID-19, is used "off-label" to treat FIP in cats.
- Different dosage : A drug is used in a different dosage or route of administration than that officially approved. For example, a drug that is only approved in tablet form could be administered as an injection.
- Other patient group : A drug approved only for adults is used in children, or a drug approved for humans is used in animals.
Legal and ethical aspects - Remdesivir and FIP
Off-label use is legal, but it poses some legal and ethical challenges. Doctors and veterinarians must take responsibility for use and ensure that patients or pet owners are fully informed of the possible risks and benefits. Informed consent is crucial.
Benefits and risks
Advantages:
- Flexibility : Doctors and veterinarians can respond to new findings and individual patient needs.
- Innovations : New therapeutic approaches can be discovered and tested through off-label use.
Risks:
- Lack of data : There may be less scientific data and clinical studies to support off-label use.
- Legal liability : In the event of side effects or failures, there could be legal consequences.
Off-label use is an important part of medical practice that makes it possible to respond flexibly and innovatively to individual health needs. However, it requires careful consideration and comprehensive information from patients or pet owners.
Summary of the Remdesivir and FIP study
Introduction - Remdesivir and FIP
Feline Infectious Peritonitis (FIP) is a serious and often fatal disease in cats caused by the feline coronavirus (FCoV). Treatment options have long been limited, but in recent years remdesivir has emerged as a promising option. This summary highlights the importance of remdesivir and FIP.
Remdesivir and FIP: How they work
Remdesivir is an antiviral drug originally developed to treat Ebola and later used to treat COVID-19 in humans. It works by inhibiting the virus's RNA polymerase, effectively stopping viral replication. Remdesivir has been shown to be effective in the treatment of FIP as it inhibits the replication of the feline coronavirus.
Studies and clinical experiences - Remdesivir and FIP
Several studies have shown that remdesivir has high effectiveness in treating FIP. In clinical studies, cats treated with remdesivir showed significant improvements in their health. Remdesivir and FIP have therefore become an important topic in veterinary medicine.
Treatment protocols - Remdesivir and FIP
Treatment of FIP with remdesivir is usually given for a period of at least 84 days. Initial therapy often begins with intravenous administration, particularly in acute cases or if the cat cannot tolerate oral medication. After stabilization, the patient can switch to oral administration of GS-441524, a related drug. This protocol has proven successful in many cases and shows how important remdesivir and FIP are in modern veterinary medicine.
Challenges and legal aspects - Remdesivir and FIP
Although remdesivir shows promising results in treating FIP, there are some challenges. In many countries, including Germany, remdesivir is not officially approved for the treatment of FIP in cats. The use is "off-label", meaning that veterinarians use the drug despite the lack of specific approval. This requires informed consent from pet owners and close monitoring of treatment. The legal and ethical implications of remdesivir and FIP are therefore important considerations for veterinarians and cat owners.
Side effects and risks - Remdesivir and FIP
As with all medications, side effects can occur when treating FIP with remdesivir. The most common include local reactions at the injection site, digestive problems and changes in liver and kidney values. It is important to monitor these possible side effects and adjust treatment accordingly. Remdesivir and FIP therefore require careful and responsible use.
Success Stories - Remdesivir and FIP
Many cat owners report impressive success treating FIP with remdesivir. Cats previously considered incurable have fully recovered and regained normal lives. These success stories underscore the importance of remdesivir and FIP in today's veterinary medicine.
Future prospects - Remdesivir and FIP
Research on remdesivir and FIP is ongoing. Scientists are continually working to develop the optimal dosages and treatment protocols. Future studies will help further improve effectiveness and make the treatment safer. Remdesivir and FIP therefore remain a dynamic and evolving field of research.
Conclusion - Remdesivir and FIP
Remdesivir and FIP have revolutionized veterinary medicine and offer affected cats a new hope. Despite legal and practical challenges, the treatment shows promising results and significantly improves the survival and quality of life of affected cats. Close collaboration between veterinarians and cat owners, as well as ongoing research, are crucial to further advance these advances.
Overall, it is clear that remdesivir and FIP are an important and future-oriented topic in veterinary medicine. The positive development and the many success stories give cause for hope and motivate further research and optimization of this treatment method.
Further information about the current study: https://www.cliniciansbrief.com/article/feline-infectious-peritonitis-treatment-outcomes?utm_medium=email&utm_source=newsletter&utm_campaign=Online+240708&oly_enc_id=4679H0135945H8Y
