Identification of genomic alterations with clinical implications in canine splenic hemangiosarcomas (HSA)
Timothy Estabrooks , Anastasia Gurinovich , Jodie Pietruska , Benjamin Lewis , Garrett Harvey , Gerald Post , Lindsay Lambert , Aubrey Miller , Lucas Rodrigues , Michelle E. White , Christina Lopes , Cheryl A. London , Kate Megquier published the following article on September 21, 2023 of your study:
https://onlinelibrary.wiley.com/doi/10.1111/vco.12925
This study examines the genomic alterations in canine splenic hemangiosarcoma (HSA), a severe form of cancer that affects endothelial cells in dogs and is characterized by its aggressiveness and short survival. The research aims to develop a fundamental understanding of the genomic landscape of HSA to develop therapeutic strategies for dogs and to gain insights into human angiosarcoma, a comparable and rare aggressive cancer.
The study is based on a sample of 109 dogs diagnosed with primary splenic HSA, all treated by splenectomy, and whose tumors were sequenced via the FidoCure® Precision Medicine Platform. Weight, age, breed, metastasis at diagnosis, and overall survival of the dogs were retrospectively evaluated.
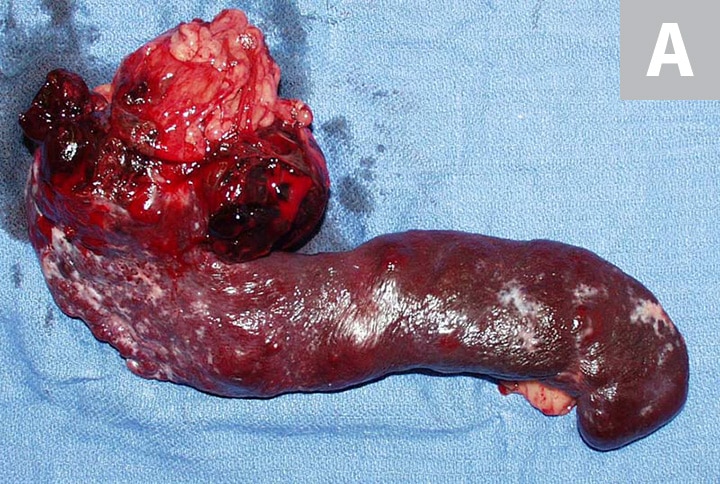
(C) https://www.cliniciansbrief.com/article/canine-hemangiosarcoma
Key findings:
Common mutations:
The research identified common somatic mutations in the TP53, NRAS and PIK3CA genes.
Relation to survival:
The study found that survival in HSA is associated with the presence of metastases at diagnosis and with germline variants in the SETD2 and NOTCH1 genes.
Relation to age and race:
Age at diagnosis correlated with somatic NRAS mutations and race. Larger dogs were more susceptible to somatic TP53 and PIK3CA mutations, while smaller dogs were more likely to have germline variants of SETD2.
Prognostic factors:
The somatic mutations and germline variants discovered have been associated with clinical variables such as age, race, and overall survival, and these genetic alterations may serve as favorable prognostic factors.
Objectives:
Development of therapeutic strategies:
A primary goal is to understand the genomic landscape of HSA to aid in the development of therapeutic strategies for dogs suffering from this cancer.
Improving precision medicine in veterinary oncology: The study aims to create a framework that leverages genomic and clinical data to provide a foundation for precision medicine in veterinary oncology.
Implications:
The study provides valuable insights into the genomic alterations and their association with clinical features in HSA in the canine spleen. These findings could aid in the development of targeted therapeutic strategies for dogs with HSA and could shed light on the genomic landscape of similar human cancers, such as: B. angiosarcomas. By understanding the relationship between genomic alterations and clinical variables, this study contributes to the exploration of prognostic factors that can potentially guide future treatments in veterinary oncology.
discussion
This study is the largest targeted sequencing study of primary canine splenic tumors (HSA) to date and identifies potential links between germline variants, somatic mutations and patient characteristics such as age, size and outcome. These data suggest that future studies could be conducted to evaluate novel treatment strategies aimed at targeting specific therapeutic vulnerabilities in HSA to improve patient outcomes.
Survive
Although different therapies could not be examined for their differential effect on survival with the available data, the median survival time (MST) of 166 days is consistent with the MSTs historically reported in splenic HSA, regardless of treatment.
Somatic mutations
Common somatic mutations in this cohort, including TP53, NRAS, PIK3CA, and PTEN, were present at similar frequencies to previous reports. We observed coexisting or mutually exclusive patterns of certain somatic mutations and germline variants, suggesting possible overlap in downstream effects. The mutations in PIK3CA and PTEN could have similar consequences, while NRAS mutations activate the RAS/RAF/MEK/ERK pathway. Furthermore, evidence suggests that knockdown of TP53 can activate RAF/MEK/ERK independently of RAS.
Overall, the patterns of coexistence/mutuality of both somatic mutations and germline variants suggest that key pathway abnormalities driving disease pathogenesis can be achieved through germline or somatic genetic modification of specific gene combinations. Therefore, a more global view of somatic and germline alterations could be informative to determine overall prognosis and develop patient-specific treatment strategies.
Mutation load
Our finding that total mutational burden correlates with somatic mutations in TP53 replicates previous published work. PIK3CA and PTEN have not yet been associated with higher mutational burden.
Germline background
Due to the high prevalence of cancer within specific dog breeds, it is believed that many breeds have established or common deleterious germline variants that predispose to cancer. Many of the common germline changes found in this study involve the RTK-RAS pathway, which is upstream of the MAPK pathway. Our results highlight the possible role of germline background in the development of HSA in different breeds as well as in outcome. Variants in SETD2 and NOTCH1 were associated with reduced OST. SETD2 is a known tumor suppressor gene, and rare germline variants in SETD2 have been associated with deficits in DNA mismatch repair in human cancer samples.
Breed-specific finds
This study found that germ cell CDKN2A variants are significantly more common in German Shepherds, suggesting that these variants may contribute to this breed's risk. The CDKN2A gene is a known tumor suppressor gene and frequent copy number deletions and losses have been documented in this breed.
The identified variant occurs in approximately 10% of dogs in a germ cell resource of 722 dogs and other canids. This variant lies at different distances downstream and upstream of the associated regions in different breeds, relative to previously described germ cell variants associated with canine osteosarcoma.
Significant differences in age and non-significant differences in overall survival (OST) were also observed between German Shepherds, Golden Retrievers and Labrador Retrievers. Because these three breeds have different average life expectancies, it is difficult to determine whether German Shepherds age more quickly or whether their strong predisposition to HSA and their tendency to have shorter OST reduce the breed's overall average survival. Golden Retrievers and Labrador Retrievers also have a high risk of HSA, so differences in genetic background and aging may play a role.
This study emphasizes the possible role of genetic variants and backgrounds in relation to the health risks and life expectancies of different dog breeds and thus provides important information for breeding and veterinary medicine.
Summary:
The retrospective study addresses the genetic background of primary splenic hemangiosarcoma (HSA) in dogs and highlights some limitations. Limitations included the inability to determine whether different treatments had a different effect on survival based on the available data.
The sampling and sequencing of the patient tumors was carried out without comparison with normal tissue, which meant that a definitive distinction between germline and somatic mutations was not possible. In addition, the study was limited to a panel of 56 genes, which is why potential drivers, copy number changes or other structural variants could not be evaluated. Non-coding variants were also not taken into account.
Breed annotations used in the analyzes were based on information provided by pet owners or veterinarians and were not confirmed by genetic data. Nevertheless, published data indicate that in most cases race information is consistent with genetic analyses.
In conclusion, this study contributes to the understanding of the genomic landscape of primary canine splenic HSA and identifies possible links between genetic background, somatic mutations and clinical variables. Further research into these compounds is necessary. Prospective work to refine the match of genomic landscapes to appropriate targeted therapeutic approaches in dogs could help improve outcomes in both dogs with HSA and humans with AS.
Another current article: https://tierarzt-karlsruhe-durlach.de/srma-beim-hund/
Abbreviations
AS angiosarcoma
CI confidence interval
CT computed tomography
HSA hemangiosarcoma
MST median survival time
NGS next-generation sequencing
OST overall survival time
WES whole exome sequencing
